Turning Seawater into Power
Turning Seawater into Power
How Saltwater Batteries are Changing the Game in Renewable Energy Storage
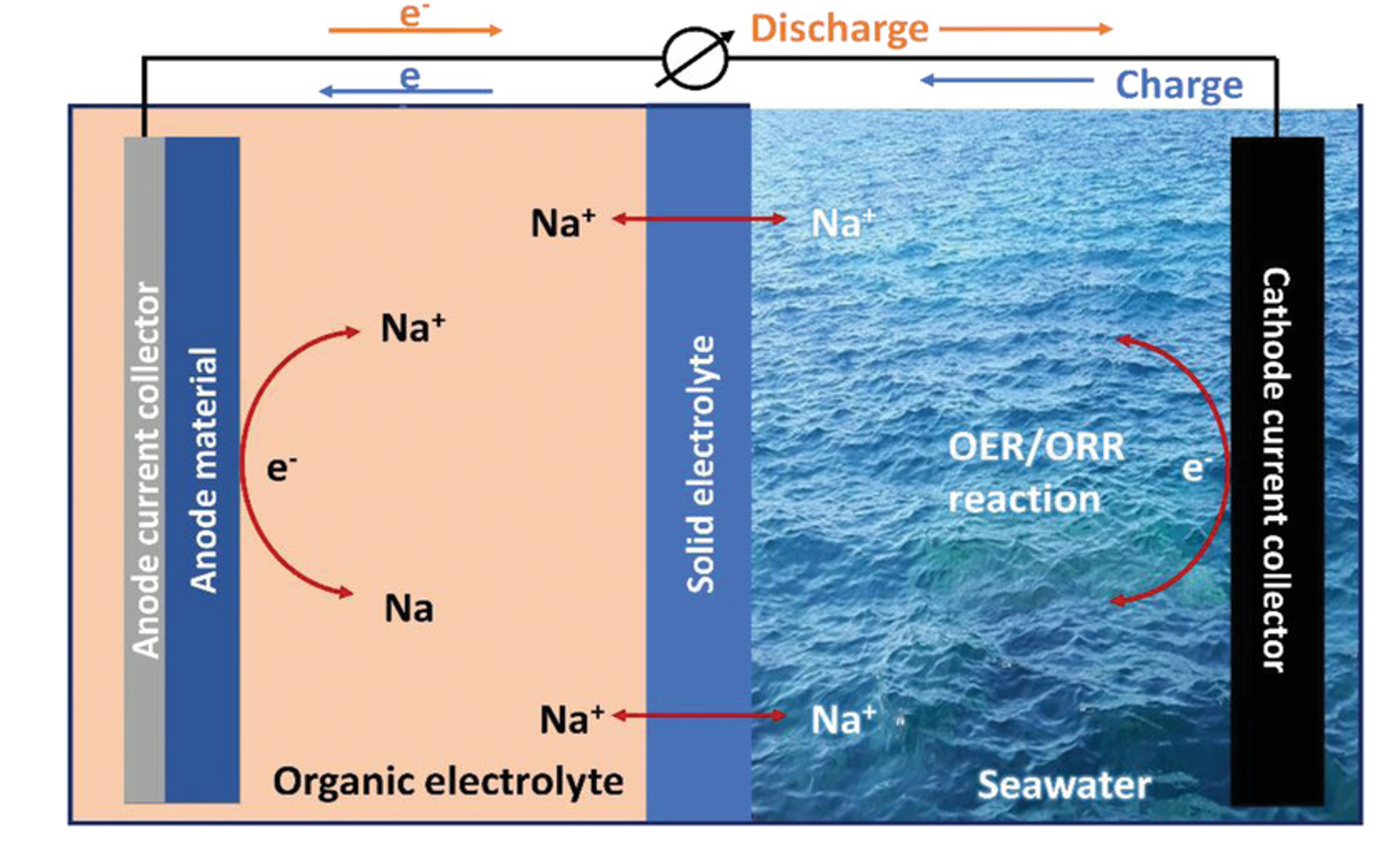
Saltwater batteries are developing as a viable alternative for sustainable energy storage, providing an innovative method to absorb, store, and discharge power from the plentiful resources available in our seas. In contrast to conventional batteries like lithium-ion or lead-acid, which depend on certain components for electrical conduction, saltwater batteries utilize sodium, the identical ingredient present in ordinary table salt. This unique chemistry differentiates them from traditional battery technologies, offering an environmentally sustainable alternative that utilizes accessible, non-toxic materials. A saltwater battery (SWB) is a distinctive energy storage technology that utilizes natural seawater as its electrolyte, rendering it a sustainable and economical option. The battery consists of two primary compartments: the anode and the cathode, which are separated by a solid electrolyte. The predominant solid electrolyte utilized in sodium-ion batteries (SWBs) is sodium superionic conductor (NASICON) ceramic, which preferentially facilitates the movement of sodium ions between the two compartments.
Mechanism of Saltwater Batteries
The novelty of saltwater batteries resides in its capacity to employ seawater as both the anode and cathode material. Sodium is extracted directly from seawater during the charging process. As the battery empties, the released sodium combines with dissolved oxygen in saltwater, serving as the oxidant to produce electricity. Essentially, saltwater supplies the critical elements required for energy storage: sodium metal for the anode and oxygen for the cathode.
More elaboratively in the anode compartment, an active metal produces electrons that traverse an external circuit to the cathode, where they react with oxygen or water, so generating an electric current. The cathode compartment, characterized by its open construction, immediately engages with saltwater, offering an essentially inexhaustible source of sodium ions. This design obviates the necessity for sodium metal electrodes or the incorporation of sodium ions, therefore streamlining the system and reducing expenses. Sodium ions are sent to the anode compartment via the ceramic electrolyte during the charging process. The open cathode configuration, submerged in seawater, enhances battery stability due to the seawater's stable temperature, which promotes effective heat dissipation and cooling. This produces a durable, low-maintenance battery characterized by a minimal self-discharge rate, prolonged longevity, and superior environmental sustainability.
Benefits Compared to Conventional Batteries
Conventional energy storage solutions, such lithium-ion batteries, depend on lithium, which is less available than sodium and necessitates ecologically detrimental mining methods. Saltwater batteries provide a sustainable alternative by utilizing salt, a more abundant and non-toxic material. The streamlined design of saltwater batteries, utilizing a liquid saltwater solution, diminishes the necessity for intricate material procurement and manufacturing procedures, rendering them a possibly more economical and eco-friendly alternative.
Utilizations and Prospective Developments
Although saltwater batteries remain in the nascent phase of research, their promise as a sustainable energy storage alternative is attracting interest. They may be especially beneficial in extensive applications, such as renewable energy storage for solar and wind power, where sustainable and scalable solutions are highly sought for. Utilizing the ocean's extensive resources, saltwater batteries may significantly contribute to the shift towards a cleaner, more sustainable energy future. The future appears promising for saltwater batteries as research and development progress. Due to their distinctive chemistry and eco-friendly benefits, they signify a prospective avenue for sustainable energy solutions and a decrease in reliance on conventional battery technology.
Recyclability
Saltwater batteries stand out due to their lack of heavy metals and toxic materials, making them easier to recycle compared to traditional battery technologies. As the global demand for batteries continues to rise, developing effective recycling strategies for used battery components will be crucial to ensuring that energy storage remains a sustainable solution. The simplicity of saltwater battery materials not only reduces the environmental impact during disposal but also streamlines the recycling process, helping to make battery technology more eco-friendly in the long run.
Future directions
Saltwater batteries represent a largely unexamined domain in energy storage, despite its promise to provide sustainable and cost-effective alternatives to conventional battery technology. These batteries employ saltwater as the principal electrolyte, harnessing a plentiful natural resource to supply the sodium ions required for electrochemical processes. The advancement and refinement of membrane separators, essential components in saltwater battery systems, can markedly improve their performance, efficiency, and durability.
In saltwater batteries, membrane separators are very important because they let ions move freely between the anode and cathode sections without getting in the way of direct contact between the electrodes. Most saltwater batteries utilize a solid electrolyte, such as sodium superionic conductor (NASICON) ceramic, for this function. This membrane permits the passage of just sodium ions, hence sustaining a stable ionic environment that facilitates efficient charge and discharge cycles. Enhancing the characteristics of these membrane separators can significantly influence the overall efficacy of saltwater batteries. Ion selectivity, conductivity, mechanical strength, chemical stability, and resistance to fouling or degradation all influence the efficiency and longevity of the battery. Improving these attributes can result in accelerated ion transfer, increased energy densities, and enhanced cycle stability, making saltwater batteries more competitive with traditional lithium-ion technology.
Amid rising demand for environmentally friendly and cost-effective energy storage technologies, saltwater batteries may play an important role in grid storage, maritime applications, and remote power systems. Continued advances in membrane separator technology will be critical to realizing their full potential. As research advances, the creation of high-performance membranes is anticipated to drive innovation, making saltwater batteries a more realistic and competitive alternative in the energy storage environment. Subsequently investing in the development of saltwater batteries and membrane technologies provides a new frontier in battery research, providing a road to sustainable energy solutions that might help a variety of businesses.
Image reproduced with permission from https://doi.org/10.1002/smll.202107913 Image credits to © 2022 The Authors. Small published by Wiley‐VCH GmbH
References and Suggested Reading
Chen, J., Sun, L., Wang, K. and Zhang, Y., 2024. Research and applications of rechargeable seawater battery. Journal of Energy Storage, 76, p.109659.
Kim, Y. and Lee, W.G., 2022. Seawater Batteries: Principles, Materials and Technology. Springer Nature.
https://bridex.fujielectric.com/salt-water-battery
Arnold, S., Wang, L. and Presser, V., 2022. Dual‐use of seawater batteries for energy storage and water desalination. Small, 18(43), p.2107913.